Bimetallic - galvanic - contact corrosion can occur when two dissimilar metals are in "electrical" contact and are connected by a conductive liquid. The resulting "cell" can lead to corrosion of one of the paired metals. Depending on the circumstances, this can be a problem when stainless steel is in contact with other metals.
Contact corrosion is a type of corrosive destruction that occurs when two dissimilar metals, i.e. metals with different electrochemical properties, come into contact.
When metals and alloys are improperly arranged, this type (kind) of corrosion damage causes many complex stainless steel structures to fail. Contact corrosion can also occur when parts of the same stainless steel come into contact with each other but are joined by welding. The weld will have different electrochemical properties than the base metal. Different mechanical treatments of stainless steel (metal) can also cause contact corrosion even in the same metal.
When two dissimilar metals come into contact, a compromise potential is realized on their surface, which differs in its value from the potentials of each metal separately. The compromise potential is determined by the intersection of the total polarization curves: anodic and cathodic.
In contact corrosion, the anode will be a metal with an electronegative potential, while the cathode has a more electropositive one. The rate of dissolution of the cathode may be higher, lower, or equal to its rate of dissolution in the same electrolyte.
The rate of anode dissolution depends greatly on the potential difference between the cathode and the anode. The processes of secondary precipitation of anode dissolution products and oxygen ionization play an important role and can have a significant impact on the course and rate of the process.
The magnitude of the compromise potential depends not only on the nature of the contacting metals, but also on the characteristics of the environment: temperature, aeration, composition of the environment, humidity, etc.
When corrosion processes occur in the soil, differential aeration pairs often occur. This is due to the uneven supply of oxygen - an oxidizing element of the environment to the surface of the metal structure. The cathode reaction proceeds with difficulty. At the same time, different free corrosion potential is observed in sections of the structure with different aeration. This is most often due to the underground metal structure lying in soils with different characteristics.
What are the causes of bimetallic - galvanic - contact corrosion ?
For this type of corrosion to occur, three elements are required:
- Two metals with different corrosion potential.
- Direct metal-to-metal electrical contact.
- A conductive electrolyte solution (such as water) must regularly connect the two metals. The electrolyte solution creates a "conductive path." This can happen through regular immersion in water, condensation, rain, fog, or other moisture sources that moisten and connect the two metals.
The further apart the metals are in terms of relative potentials, the greater the driving force in the "cell". For example, stainless steel in contact with copper presents less risk of rusting than in contact with aluminum or galvanized steel.
To complete the "cell", a conductive liquid must connect the contact metals. The more conductive the liquid, the greater the risk of corrosion. Sea water or humid air saturated with salt pose a greater risk than contact with rainwater in urban conditions.
If metals are constantly dry, bimetallic (galvanic, contact) corrosion cannot occur.
Corrosion at contact of galvanized and stainless steel
Galvanized steel in contact with stainless steel is not generally considered to pose a significant corrosion risk, except perhaps in severe (marine type) conditions and provided the zinc layer is continuous over the entire surface. In such situations, precautions such as isolation barriers are generally considered sufficient to avoid bimetallic corrosion in most practical situations.
But there is still a risk. Galvanized and stainless steel have different service lives. Sooner or later, galvanized steel will start to rust anyway, and the risk of rust spilling over onto stainless steel will still remain. In addition, there is almost no perfectly dry environment under normal conditions. There is always some percentage of moisture in the air. Therefore, it is better to play it safe and, if possible, produce structures only from stainless steel. For example, manufacturers often weld galvanized flanges made of ordinary metal to railing posts. Subsequently, this combination leads to the formation of rust "with its leakage from the outside."
Corrosion at contact of aluminum and stainless steel
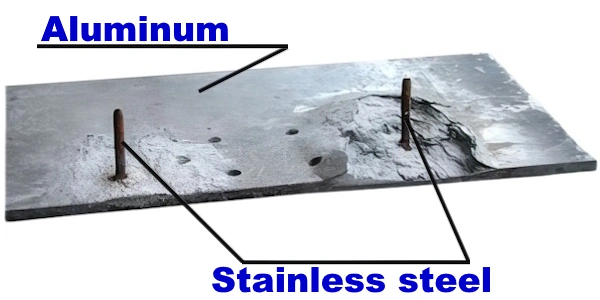 Aluminum and stainless steel together also pose a risk of bimetallic corrosion. In this combination, the influence of relative surface area on corrosion is important.
Aluminum and stainless steel together also pose a risk of bimetallic corrosion. In this combination, the influence of relative surface area on corrosion is important.
A large "cathode" area relative to the "anode" will accelerate anodic corrosion. Although aluminum is anodic to stainless steel, large relative surface areas of aluminum relative to stainless steel may be acceptable depending on local conditions.
Stainless steel fasteners in aluminum plates or sheets are generally considered safe, whereas aluminum rivets or bolts fastening stainless steel parts are an unwise combination since there is a practical risk of corrosion.
An example of safe use of stainless steel and aluminum together is the use of stainless steel fasteners and hold-down bolts to secure aluminum roadway or bridge fencing. Even without insulation between the metals, the risk of corrosion should be low.
In contrast, in a marine environment, severe localized pitting of aluminum steps was observed when non-insulated stainless steel bolts were used to hold the ladder in place. However, on the same ladder, bolts with sound-insulating washers showed no pitting of the surrounding aluminum. This illustrates the positive effect of breaking up a corrosion pit by isolating two "dissimilar" metals in extreme cases.
Discoloration of stainless steel by corrosion products
The problem may be staining of stainless steels from corrosion products of the bonded metal. Lead and copper are close enough in rating to stainless steel that the risk of bimetallic corrosion should be low. However, any corrosion product that is washed onto stainless steel may cause problems unrelated to the bimetallic effect, so these cannot be predicted.
So, galvanic corrosion of stainless steel is a corrosion damage that occurs when two dissimilar metals form an electrically conductive junction and encounter a common corrosive electrolyte. The less noble of the two metals will dissolve (anodic metal dissolution reaction), and the more noble metal will not corrode (it only acts as a cathode for oxygen reduction).
In galvanic corrosion, the corrosion rate of a less noble metal will be higher than that of the same metal in a free corrosive environment in the absence of contact with another metal.
Using thermodynamic data and taking into account general experience gained in similar situations, it is possible to predict which material combinations with stainless steel will be susceptible to galvanic corrosion.
Unfortunately, all sorts of mistakes and blunders are often made in assembly and construction work. The consequences of some of them may become apparent over a short period of time. As for the topic of stainless steel fasteners and rigging, the most common mistake in its application is undoubtedly an attempt to "combine the incompatible", namely to assemble stainless steel and elements made of ordinary steel into one unit.
And in such situations we can clearly observe rapid and merciless contact corrosion. This type of corrosion develops when metals with different electrochemical properties come into contact. In scientific terms, during contact corrosion, a compromise potential is realized on the surface of both components of the system, determined by the cross-section of the total anodic and cathodic polarization curves. The dissolution rates of both components of the system at this potential will differ from the individual dissolution rates of each of the components of the same solution. To put it simply, the junction of stainless and carbon alloys will immediately begin to spread rust across both products, and the rate of this corrosion will significantly exceed the normal rust rates of each metal.
Methods of protection against contact - bimetallic - galvanic corrosion
The occurrence of contact - bimetallic - galvanic corrosion can be prevented by using insulation between two metals, using paint or metal coatings, as well as by using intermediate parts that are neutral to such corrosion.
Corrosion-resistant stainless steels and alloys, when used in medium and severe conditions, are well combined with the same steels, with titanium and its alloys, with chromium corrosion-resistant steels, with nickel and its coatings, with copper and its alloys, with chromium and its coating, with silver and its coating on copper, with gold, platinum, palladium and rhodium (metal and coating).
The best protection is to make, if possible, the entire structure only from stainless steel and of one grade.
How to remove bimetallic - galvanic - contact corrosion / rust and protect stainless metal from destruction ?
When corrosion occurs on stainless alloy products, it is not a good sign. After all, rust not only has an ugly appearance, but also destroys the stainless metal itself. Therefore, it is necessary to take timely measures to avoid this error and its consequences.
Bimetallic - galvanic - contact corrosion at the initial stage of its development is not as terrible as other types of corrosion of stainless steel. And while it has not penetrated deep enough into the metal - it can be easily removed using conventional traditional methods and methods of processing and cleaning stainless steel:
- Chemical method: etching paste, acids, special gels, liquids, gel-like agents or aerosols for removing rust from the surface of stainless steel.
- Mechanical cleaning: grinding, polishing, glass blasting, water blasting, etc.
- Electrochemical cleaning: surface rust is removed fairly easily using electric current and electrolytic liquids. Electrochemical polishing: done in baths filled with electrolyte.
The most effective cleaning method will be to use several methods. Thus, mechanical polishing will show the best result if applied after chemical or electrochemical cleaning.
Bimetallic - galvanic - contact corrosion, even after rust has been removed from the surface of stainless metal, can manifest itself again and again if the causes of its manifestation have not been completely eliminated. If the surface has been poorly cleaned of rust (rust remains on the surface), then under favorable corrosion conditions (for example, moisture), rust can appear again. Therefore, cleaning should be carried out very carefully, observing all the established norms and rules of a particular removal agent. And the cause of this type of rust must be completely eliminated.
