Intergranular corrosion of stainless steel, unlike many other forms of stainless steel corrosion, occurs at the microscopic level, affecting the structure of the metal. Signs of damage do not always appear on the surface of the metal. The occurrence of intergranular corrosion requires certain circumstances, and in some cases the damage caused by it can be avoided.
Let's take a closer look at what intergranular corrosion is, when and how it occurs, and the best methods for preventing it and minimizing the damage it causes.
What is intergranular corrosion of stainless steel ?
Stainless steel, like many other metals and alloys, has a crystalline structure. This means that the metal itself is made up of grains of varying sizes. Where these grains meet is called the grain limits.
Intergranular corrosion of stainless steel, also known as weld decay, attacks these limits, causing damage to the metal at the molecular level. Cracking and grain loss can occur, resulting in decreased structural integrity, reduced pressure-bearing capacity, and further promoting additional corrosion.
Like stress corrosion cracking, this can occur with little or no visual evidence of corrosion attack.
Thus, ignoring the risks of intergranular corrosion can lead to catastrophic failure or partial damage to pipelines, individual parts, structures and components made of stainless steel.
What causes intergranular corrosion of stainless steel ?
Intergranular corrosion of stainless steel occurs when metals of certain stainless steel grades and alloys reach temperatures between 425 °C and 870 °C. Such temperatures are most common during welding, heat treating, or service/work in high-temperature environments.
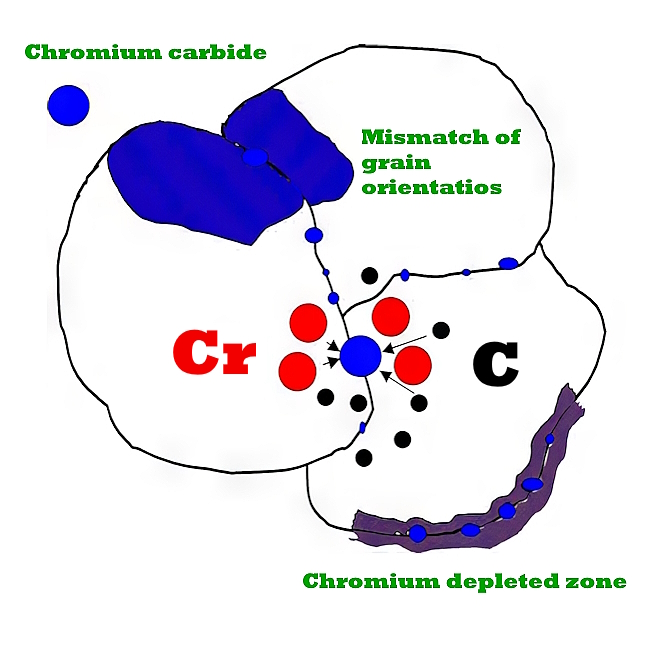
When stainless steel withstands these extreme temperatures, it changes at the structural level. The chromium present in the alloy reacts with the carbon to form chromium carbide near the grain boundaries. This carbide formation essentially turns the boundaries into anodic cells. The internal grains then function as cathodic cells, and the process of intergranular corrosion begins.
Prevention and elimination of damage caused by intergranular corrosion
How to prevent intergranular corrosion ?
Proper material selection is an important element of risk mitigation and ensures the long-term safety and performance of stainless steel structures and components. When looking for stainless steel grades with excellent intergranular corrosion resistance, pay particular attention to low-carbon alloys, which are often designated with the letter L (AISI 316L, AISI 317L, etc.).
When selecting low carbon stainless alloys, options with carbon content below 0.03 percent ensure that there is not enough carbon to form carbides.
If low-carbon stainless alloys are not suitable for your intended application, alloys with added titanium or niobium provide exceptional resistance to intergranular corrosion. However, alloys with added titanium or niobium are quite susceptible to a special form of intergranular corrosion called knife corrosion.
What is knife corrosion of stainless steel ?
Knife corrosion is a form of intergranular corrosion of a stainless metal/alloy, usually stabilized stainless steel, along the abutment or contact line with a weld, after heating to the sensitization temperature. Knife corrosion occurs when carbon reacts with titanium or niobium instead of chromium.
The corrosion action is limited to a very narrow line adjacent to the melting line. Visually, such damage appears sharp as a razor (hence the name of the corrosion "knife line", "knife corrosion"). Visually, the appearance of knife corrosion can be recognized if the lines have already formed along the weld.
Why does knife corrosion occur ?
For stabilized stainless steels and alloys, the carbon is bound by stabilizers (Ti or Nb), and during welding, no decomposition of the weld occurs in the heat-affected zone. However, during further heat treatment or welding, chromium carbide may be released, leaving a narrow band adjacent to the melting line, susceptible to intercrystalline corrosion.
It is possible to prevent intergranular corrosion, including knife corrosion. Heat treatment can often solve the problem and return the metal structure to near its original state. In some cases, solution annealing (also known as quench annealing or solution hardening) is an effective means of eliminating intergranular corrosion damage in austenitic stainless steels. The process involves heating the metal to temperatures between 1060 °C and 1120 °C. After heating, the stainless metal is calcined with water, rapidly cooling it and setting the grain and structure.
Unfortunately, the heat treatment method is not ideal for treating large structures or parts. In addition, it does not protect pipes or other components from damage when re-welded for repairs.
International standards such as ASTM offer long-established standards to help determine the susceptibility of each alloy, stainless steel grade or component to intergranular corrosion.
Intergranular corrosion of austenitic stainless steels
For austenitic stainless steels, intergranular damage is usually the result of precipitation of chromium carbides (Cr23C6) at the grain boundary, which creates a narrow zone of chromium depletion at the grain boundary. This condition is called sensitization. Sensitization involves precipitation of chromium carbides at the grain boundary, which results in a narrow zone of chromium depletion at the grain boundary.
Since chromium is the main alloying element that makes stainless steel resistant to corrosion, areas with low chromium content are susceptible to corrosion advantage. It is believed that this occurs because the chromium content immediately adjacent to the carbide may be lower than that required for the stainless steel alloy. If the carbides form a continuous network at the grain boundary, corrosion can lead to delamination or rupture at the boundary and possible grain drop or loss.
Precipitation of chromium carbide in austenitic stainless steels
Chromium carbides tend to precipitate within the grains of austenitic stainless steels in the temperature range of 510 °C to 790 °C. Any exposure or temperature transition into this temperature range during production, fabrication or servicing of the metal can potentially increase the sensitivity of the stainless steel.
Conventional techniques such as welding, stress relieving, and hot forming can expose austenitic stainless steel to a sensitizing temperature range. The formation of chromium carbides is easily reversed by solution annealing heat treatment. The test methods outlined in ASTM A262 have been developed to detect the susceptibility of austenitic stainless steels to intergranular fracture.
The time and temperature required to create a tendency for intergranular fracture depend on the composition of the alloy, particularly its carbon content.
Three approaches have been used with austenitic stainless steels to minimize the effects of intergranular corrosion. The sensitized material can be solution annealed by heating to a temperature at which the carbides are dissolved and the chromium-depleted regions are removed. The carbon is then held in solution by rapid cooling through the sensitizing temperature range. The recommended solution annealing temperature varies with the alloy and is typically in the range of 1040 °C to 1180 °C followed by rapid cooling.
Resistance to intergranular corrosion can also be achieved by reducing the carbon content to below 0.030%. Low carbon grades such as AISI 304L, 316L and 317L were developed to resist sensitization during typical welding processes, but they do not resist sensitization under long-term exposure in the critical service temperature range. Highly alloyed and more corrosion-resistant stainless steels such as AISI 904L have very low carbon content and susceptibility to intergranular corrosion is not usually a concern.
The addition of stabilizing elements such as Ti, Nb(Cb), and Ta can also provide increased resistance to sensitization, especially during long-term exposure in the critical service range. These stabilizing elements tend to form carbides that are more stable than chromium carbide in the temperature range of 1230 °C to 790 °C. Thus, when the alloy is cooled from high temperatures, the carbon combines with the stabilizing elements and becomes unavailable for chromium carbide precipitation in the lower sensitizing temperature range of 510 °C to 790 °C. Common stabilized austenitic grades include types such as AISI 321, 347, and 316Ti.
For stabilized grades of stainless steel, standard solution annealing procedures do not usually bind all of the available carbon. Thus, when solution annealed stabilized grades are exposed to the sensitizing temperature range (790 °C to 510 °C) for long periods, chromium carbide precipitation and sensitization can occur. Stabilizing heat treatments can be used to more effectively bind carbon by completing the precipitation reactions. These treatments consist of holding the alloy for several hours at temperatures between 820 °C and 870 °C.
Intergranular corrosion of ferritic stainless steels
Although the intergranular fracture of ferritic stainless steels is similar to that of austenitic stainless steels, there are some important differences. Because the solubility of nitrogen in the ferrite crystal structure is low, the precipitates that cause sensitization of ferritic grades include both chromium carbides (Cr23C6) and chromium nitrides (Cr2N).
In ferritic stainless steels, sensitization occurs upon cooling from higher temperatures (930 °C). At these high temperatures, carbides and nitrides go into solution and may precipitate at grain boundaries during cooling, resulting in chromium depletion. The very high diffusion rates in the ferritic structure make it impossible to cool the steel fast enough to avoid precipitation of carbides and nitrides within the grains. Therefore, most commercial ferrite grades avoid sensitization by limiting the levels of C and N and requiring the addition of stabilizing elements such as Ti, Ta, or Nb.
If sensitization has occurred in a ferritic stainless steel, the condition can be cured by back-diffusion of chromium into the depleted areas. "Cure" can be achieved by holding the material at 590 °C - 650 °C for several hours. Test methods outlined in ASTM A763 have been developed to detect the susceptibility to intergranular fracture in ferritic stainless steels.
Intergranular corrosion of martensitic stainless steels
Most publications devoted to intercrystalline corrosion relate to austenitic steels. Studies of intercrystalline corrosion of martensitic stainless steels in modern technical literature are practically absent or very few.
Intergranular corrosion is a potential problem for these materials because martensitic stainless steels are almost always used in the tempered condition, which precipitates carbides. General methods for testing the susceptibility of stainless steels to intergranular attack are described in ASTM A2621 for austenitic steels and ASTM A7632 for ferritic steels.
Intergranular corrosion of duplex stainless steels
Duplex stainless steels as an alternative to conventional austenitic stainless steels are becoming increasingly common, particularly for applications in sour environments where high corrosion/stress corrosion cracking resistance is required in aggressive chloride/sulfide environments.
Although these steels have many excellent properties, there are limitations associated with welding these steels, particularly in controlling the weld structure and properties and understanding how weld metallurgy can affect susceptibility to intergranular corrosion.
Key findings
- Intergranular corrosion of stainless steel (also known as stainless steel weld decay) affects stainless steel at a structural level and may not show visible signs of damage until corrosion has progressed significantly.
- Welding, improper heat treatment and exposure to temperatures from 425 °C to 870 °C are necessary to initiate the intergranular corrosion process.
- Selecting low-carbon stainless alloys and grades or alloys with added titanium or niobium can significantly improve resistance to intergranular corrosion.
- When selecting a low carbon alloy / grade, options with a carbon content of 0.03% or less are recommended.
- While solution annealing and heat treatment may offer options for eliminating or mitigating intergranular corrosion damage, they often do not protect against future damage. They may have limited success in reversing corrosion depending on the severity of the corrosion process.